Abstract

Background: Congenital thrombotic thrombocytopenia purpura (cTTP) is an ultra-rare disorder resulting from an inherited deficiency of ADAMTS13, a von Willebrand factor (VWF)-cleaving metalloprotease. Clinically, cTTP is characterized by recurring, and potentially life-threatening, episodes of VWF-platelet aggregation and microvascular thrombosis. Treatment has traditionally been replacement of ADAMTS13 using fresh frozen plasma (FFP) or solvent detergent treated plasma, options which are limited by risks of severe allergic reactions, viral exposure, and volume overload. The marketed plasma-derived factor VIII/VWF Koate® (hereafter, FVIII/VWFKoate) has been shown to have the highest concentration of ADAMTS13 among tested plasma-derived concentrates [Peyvandi F, et al. Am J Hematol. 2013;88:895-898] and can be administered by the patient or a caregiver in the patient's home. Based on a prior case series that reported safe and effective use of FVIII/VWFKoate in 8 patients with cTTP [Aledort LM, et al. J Pediatr Hematol Oncol 2017;39:524-527], we conducted a retrospective study to gather additional data regarding the use of FVIII/VWFKoate for this disease.
Methods: This was a multi-center (United States, n=8 sites; Canada, n=1 site), retrospective, non-interventional chart review of patients who had received at least one dose of FVIII/VWFKoate for the management of cTTP between 8/1/2014 and 1/31/21 (through 12/31/21 at one center). Data collected included patient demographics, medical and family history relevant to cTTP, past use and tolerability of FFP, and details regarding FVIII/VWFKoate therapy, including dose, duration of therapy, TTP episodes, clinical and laboratory outcomes, adverse events (AEs), and reported difficulty with FVIII/VWFKoate administration.
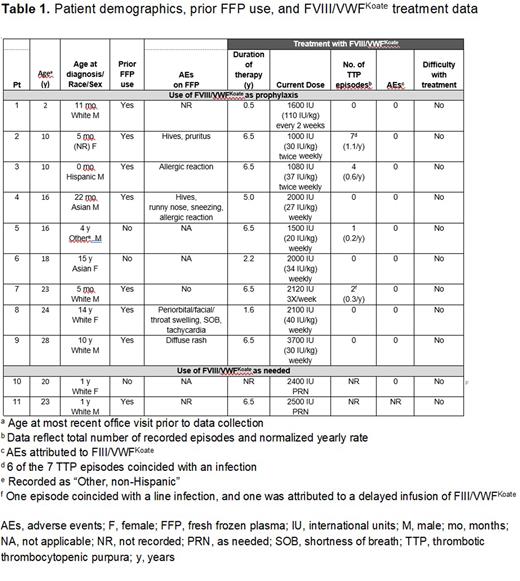
Results: The cohort included 11 patients (7 males, 4 females) with cTTP, ranging in age (at the time of data collection) from 2-28 years (Table 1). Age at diagnosis of cTTP ranged from 0 months to 15 years. Eight patients had at least one immediate family member with cTTP. Eight patients had been previously treated with FFP and 5 were noted to have experienced AEs including allergic reactions/rashes. The average duration of therapy with FVIII/VWFKoate was 4.8 years (range, 0.5-6.5 years). Individual FVIII/VWFKoate doses ranged from 1000-3700 IU per dose. Nine patients used FVIII/VWFKoate regularly as prophylaxis; dosing frequencies at the time of data collection included weekly (n=5), twice weekly (n=2), 3 times/week (n=1), and every 2 weeks (n=1). Two patients used FVIII/VWFKoate only as needed. Doses were administered by the patient/caregiver (n=9) or by a nurse (n=2). Among the 9 patients using FVIII/VWFKoate on an ongoing basis as prophylaxis, the normalized annual rate of breakthrough TTP episodes ranged from 0.2 to 1.1 episodes/year. There were no AEs attributed to FVIII/VWFKoate. At the time of cTTP diagnosis, 4 patients had microangiopathic hemolytic anemia, and 3 of the 4 did not have persistent anemia following treatment with FVIII/VWFKoate; 1 additional patient had persistent anemia considered unrelated to cTTP. Among 8 patients who used FVIII/VWFKoate as prophylaxis and who had baseline data regarding presence/absence of thrombocytopenia, all 8 had thrombocytopenia prior to FVIII/VWFKoate and 7 of the 8 (87.5%) did not have thrombocytopenia after using FVIII/VWFKoate (95% confidence interval, 46.7% to 99.3%). The 1 patient who had residual thrombocytopenia after using FVIII/VWFKoate had no recorded TTP episodes while using FVIII/VWFKoate. Renal impairment was noted in 1 patient at the time of diagnosis and 1 (different) patient following FVIII/VWFKoate treatment. None of the patients were noted to have evidence of residual CNS impairment at the time of diagnosis or following FVIII/VWFKoate treatment.
Conclusions: These data, reflecting treatment periods averaging almost 5 years, suggest that FVIII/VWFKoate appears to be a safe and effective source of the missing ADAMTS13 enzyme in patients with cTTP, producing a marked reduction in prevalence of thrombocytopenia, low frequency of TTP episodes, and with the added benefit of self- or caregiver-administration. Regimens of FVIII/VWFKoate varied greatly in this real-world study, and additional experience is needed to optimize dosing recommendations for this rare disease.
Disclosures
Chrisentery-Singleton:Hema Biologics: Consultancy, Honoraria, Speakers Bureau; Octapharma: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Biomarin: Consultancy, Honoraria, Speakers Bureau; CSL Behering: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy; Genentech: Consultancy, Honoraria, Speakers Bureau; Kedrion: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Speakers Bureau; GBT: Consultancy, Honoraria, Speakers Bureau. Ibrahimi:Karyopharm Theraputics: Divested equity in a private or publicly-traded company in the past 24 months. Khan:Genentech: Consultancy; Biomarin: Consultancy; Kedrion: Consultancy; Takeda: Consultancy; Novo Nordisk: Consultancy; Bayer: Consultancy. Mahajerin:Spark: Honoraria, Speakers Bureau; Alexion: Honoraria, Speakers Bureau; Genentech: Honoraria, Speakers Bureau. Rajasekhar:Alnylam: Other: Addi, Research Funding; Sanofi: Research Funding; Shire/Takeda: Research Funding; Biomarin: Research Funding; Dimensions Therapeutics: Research Funding; Genentech: Research Funding; Janssen Pharmaceuticals: Research Funding; Roche: Research Funding; Bio Products Laboratory: Research Funding; LTD: Research Funding; Kedrion: Research Funding. Sharma:Alexion: Speakers Bureau; Bayer: Consultancy; BMS: Research Funding; HEMA Biologics: Consultancy; Sanofi Genzyme: Consultancy, Speakers Bureau; Novo Nordisk: Speakers Bureau; Biomarin: Research Funding, Speakers Bureau; Astra Zeneca: Research Funding. Steele:Canadian Blood Services: Honoraria. Carpenter:genentech: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal